Health Technology Assessment (HTA)
Health Technology Assessment (HTA) is a multidisciplinary process that uses explicit methods to determine a health technology’s value for healthcare decision-making. Attitudes towards HTA vary globally, with some countries using a variety of methods and analyses and others hardly any.
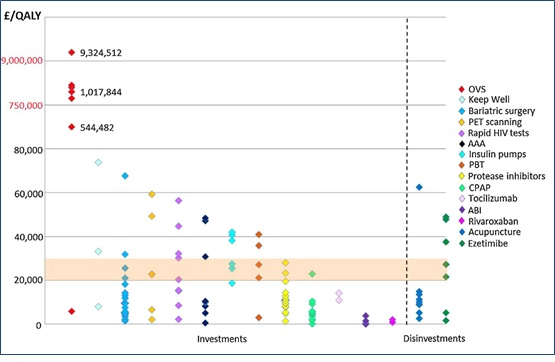
Recently Published: Local Health Care Expenditure Plans and their Opportunity Costs
8 September 2015
Recently published in Health Policy is an article by Sarah Karlsberg Schaffer, Jon Sussex, Nancy Devlin and Andrew Walker entitled “Local health care expenditure plans and…

Incorporating Life-cycle Price Modelling into Pharmaceutical Cost-effectiveness Evaluations
25 August 2015
A Research Paper on the topic of incorporating life-cycle price modelling into pharmaceutical cost-effectiveness evaluations has just been published by OHE.

Recently Published: Payer Perspectives on Future Acceptability of Comparative Effectiveness and Relative Effectiveness Research
18 August 2015
This paper examines the views of US and European payers on the rapidly evolving fields of comparative effectiveness and relative effectiveness research.

Spotlight on OHE: Value Footprint of Oncology Treatments, Treating Obstructive Sleep Apnoea and Sources of Motivation in Health Care Organisations
4 August 2015
The blog provides links to slide sets and posters relating to the value footprint of oncology treatments, treating obstructive sleep apnoea and sources of motivation in…

An Issues Panel: Developing Cost Effectiveness for Decision Making: What can be Learnt from “Value Based Pricing”?
29 July 2015
This post reports on an Issues Panel which was held at the Spanish Health Economics Association Annual Conference which took place in Granada, Spain 17-19 June…

Guest post: The Economics of Elevated Hospital Mortality at Weekends
14 July 2015
Rachel Meacock reports on the economics of elevated hospital mortality at weekends.

Patient-reported outcomes measures (PROMs) in Health Economics – an Overview and Key Developments in the Field
30 June 2015
In June 2015 OHE’s Nancy Devlin gave a seminar at the Royal Statistical Society on the topic of patient-reported outcomes measures (PROMs) in health economics –…

OHE at HTAi Oslo: A New Drug Development Paradigm, and the Effect of NICE Decisions Abroad
19 June 2015
This post summarises OHE’s activities at this year HTAi meeting in Oslo, June 2015. Topics include the effect of NICE decisions abroad and a new drug…

OHE at HTAi Oslo: A New Drug Development Paradigm, a Framework for Developing Formularies in MICs, and the Effect of NICE Decisions Abroad
15 June 2015
This post summarises OHE’s activities at the upcoming HTAi meeting in Oslo, June 2015. Topics include the effect of NICE decisions abroad, a framework for developing…