Sign up to our newsletter Subscribe
Multi-Indication Pricing (MIP): Practical Solutions and Steps to Move Forward
Sign up to our newsletter Subscribe


This report analyses patient access in the UK to new medicines authorised via the centralised procedure between 2011 and 2016.
The centralised procedure was created in 1995 to facilitate access to innovative medicines across the European Union (EU). Since then the scope of authorisation via the centralised procedure has been broadened and made mandatory for orphan and oncology medicines.
We analysed routine funding in the NHS for new medicines recently authorised via the centralised procedure with a particular focus on oncology and orphan medicines. We utilised a database of outcomes of health technology assessment (HTA) evaluations conducted in the UK by NICE, AWMSG and SMC: OHE’s Medicines Tracker. We considered centrally authorised products (CAP) approved between 1 January 2011 and 31 December 2016.
Our analysis revealed that:
Our analysis found that in England and Scotland, products used in oncology and orphan disease were associated with a lower odds of receiving a positive HTA recommendation compared with non-oncology products and products which did not receive an orphan designation in the EU.
An analysis of time elapsed between marking authorisation and the publication of the final HTA recommendation found the median time to be five months in Wales, eight months in Scotland, and 13 months in England. Notably, the evaluation of orphan or oncology medicines, used in therapeutic areas with recognised unmet medical needs, was not consistently prioritised by HTA agencies in the UK. Additionally, we found little agreement in the recommendations resulting from the evaluations completed by AWMSG, NICE and SMC.
The availability of and routine funding of medicines in England according to three main therapeutic classes (oncology, anti-infectives and other) from authorisation via the centralised procedure to access in the NHS is presented in Figure 1.
Figure 1: From authorisation to availability in the NHS in England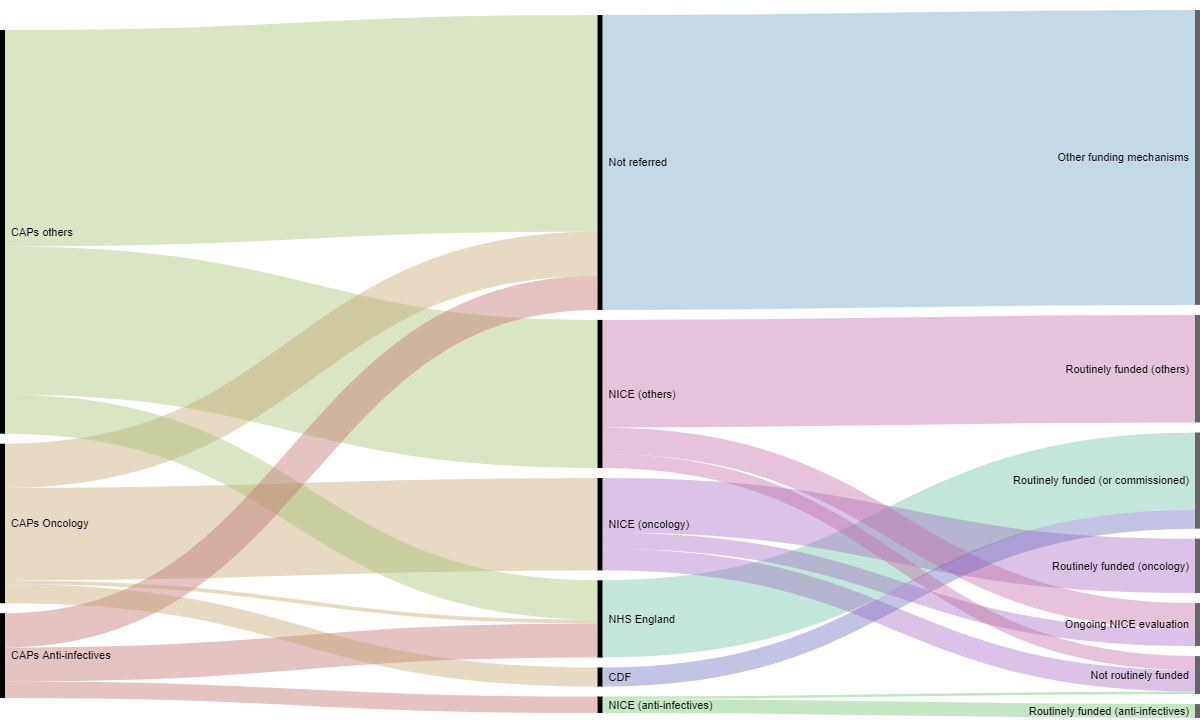
Some products are directly commissioned by the NHS and do not go through NICE’s evaluation process. For the period of interest this includes 61 products directly funded by NHS England specialised commissioning. We identified 25 orphan medicinal products that were directly commissioned by NHS England. Additionally despite the fact that the NHS has put several mechanisms in place to grant access to orphan drugs in England, only 60% of the orphan drugs which received a marketing authorisation during the period of our study were accessible to patients in England (this confirms the results of previous OHE research).
The availability of and routine funding of orphan medicines in England from authorisation via the centralised procedure to access in the NHS is presented in Figure 2.
Figure 2: Orphans vs non-orphans, from authorisation to availability in the NHS in England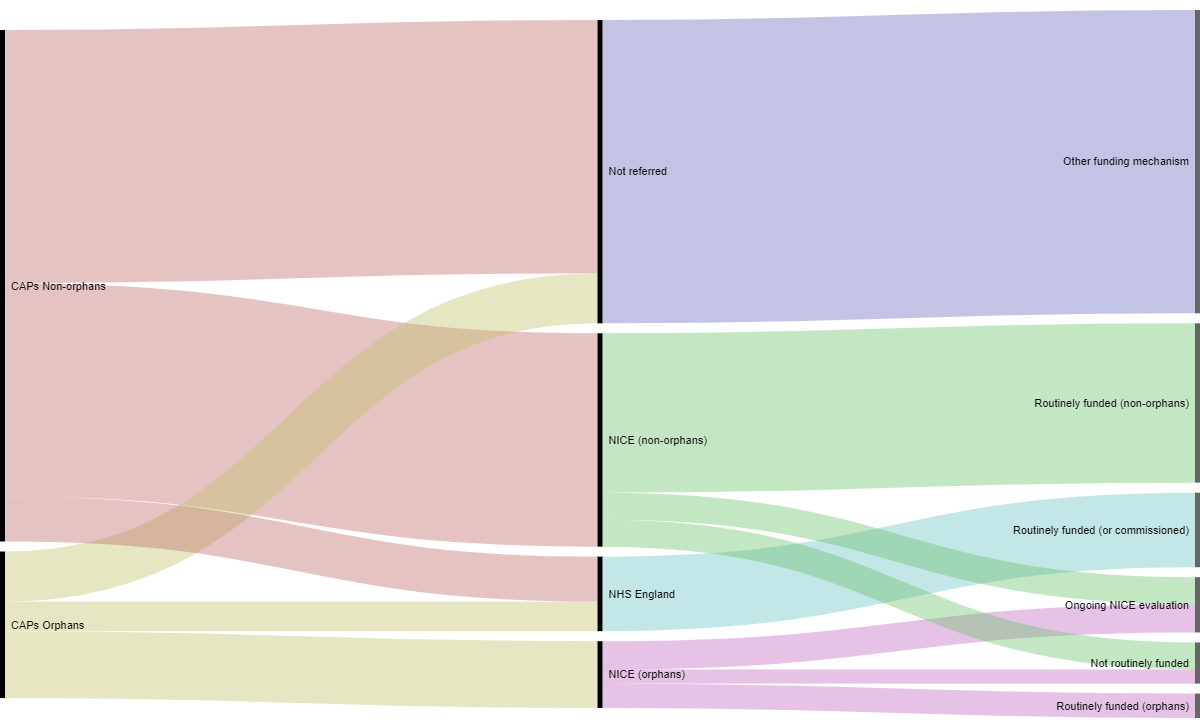
In summary we find that a substantial number of products which received an EU authorisation between 2011 and 2016 were not referred for a HTA evaluation in the UK. Previous evidence suggests that before 2011 oncology medicines were more likely to receive a positive recommendation than products for the other classes (Dakin et al. 2015). Our study shows that this likelihood has been reversed since 2012.
We also show that there is both variation across agencies and variation across therapeutic classes in terms of adoption decisions and access across the three devolved nations.
Access the full report here.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!