Health Technology Assessment (HTA)
Health Technology Assessment (HTA) is a multidisciplinary process that uses explicit methods to determine a health technology’s value for healthcare decision-making. Attitudes towards HTA vary globally, with some countries using a variety of methods and analyses and others hardly any.
Bridging the Gap: Pathways for Regulatory and Health Technology Assessment of Histology Independent Therapies
8 January 2020
Histology independent therapies, a new class of medicines that target cancer based on specific genomic or molecular alterations of cancer cells rather than tissue of origin,…
International Cost-Effectiveness Thresholds and Modifiers for HTA Decision Making
5 January 2020
OHE presents an overview on the use of cost-effectiveness thresholds (CETs) in a number of selected countries in their decision-making process for health technology assessments (HTAs).…

Are Cost-Effectiveness Thresholds fit for Purpose for Real-World Decision Making?
1 February 2020
Cost-Effectiveness Thresholds (CETs) are used in a selected number of countries as tool in decision-making on funding and reimbursements for new healthcare technologies.

Ethical and Economic Issues in the Appraisal of Medicines for Ultra-Rare Conditions
1 January 2020
In light of concerns that not all medicines for ultra-rare (also known as ultra-orphan) conditions are appraised under the same NICE process, a new OHE Consulting…
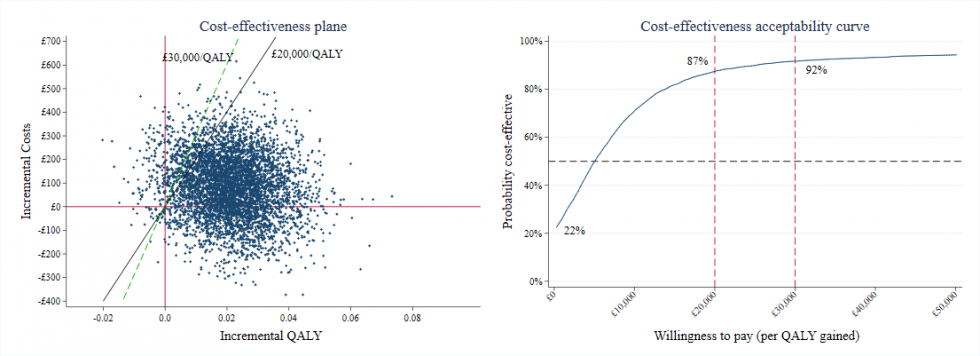
25 Years of the Cost Effectiveness Acceptability Curve
30 December 2019
In 1994 Ben van Hout introduced the concept of the cost effectiveness acceptability curve (CEAC). In the 25 years since, the CEAC has become a standard…

OHE Presents at Global AMR R&D Hub Board of Members Meeting in Paris
18 December 2019
OHE presented at the Global AMR R&D Hub Board of Members Meeting in Paris, on adapting HTA and payment mechanisms to incentivise new drugs to tackle…

HTA and Payment Mechanisms for New Drugs to Tackle AMR
23 September 2019
A new OHE Research Paper summarises the findings of a project funded by the Wellcome Trust on innovative HTA methods and contracting for antibiotics. The paper…

OHE Lunchtime Seminar: How Do We Measure the ‘Value’ in Value-based Care?
19 August 2019
OHE Lunchtime Seminar with Steve McKenna, 17th September 2019. The seminar will examine conceptual and practical aspects of measuring value in health care.

Assessing the Life-cycle Value of Second-generation Antipsychotics in Sweden and the UK
17 July 2019
This research by a team of OHE and IHE researchers estimates the value added by second generation antipsychotics over their life-cycle in the UK and Sweden.…