Sign up to our newsletter Subscribe
Cornerstones of “Fair” Drug Coverage: Appropriate Cost – Sharing and Utilization Management Policies for Pharmaceuticals

Sign up to our newsletter Subscribe



In November 2016 Professor Dame Sally Davies delivered the 23rd OHE Annual Lecture on the topic of: Ten Years of the NIHR: Achievements and Challenges for the Next Decade. The lecture is now available as a publication, available for download.
In November 2016 Professor Dame Sally Davies delivered the 23rd OHE Annual Lecture on the topic of: Ten Years of the NIHR: Achievements and Challenges for the Next Decade. The lecture is now available as a publication, available for download here.
The National Institute for Health Research (NIHR), created in April 2006, is a “virtual” organisation often referred to as the research arm of the NHS. It funds health and care research in the UK, translating discoveries into practical products, treatments, devices and procedures, involving patients and the public in all its work.
The figure below is a schematic of how the NIHR system is intended to work: each part of the system supports the other, ultimately translating the knowledge from basic research into benefits for patients.
Figure 1: NIHR: A Health Research System
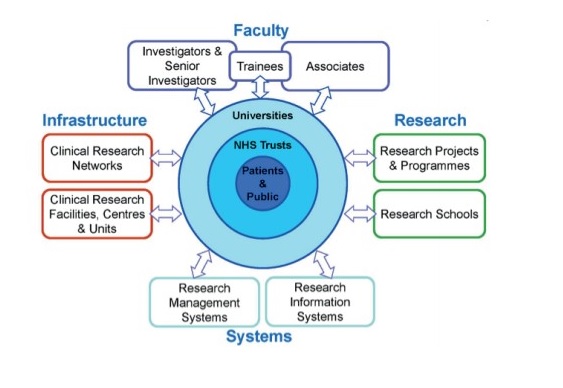
Source: NIHR, 2016.
Dame Sally Davies and Dr Russell Hamilton were the driving forces behind creation of the NIHR.
Through the course of the OHE Annual Lecture Dame Sally:
Dame Sally was appointed Chief Medical Officer for England and Chief Medical Advisor to the UK Government in March 2011. Dame Sally is an independent advisor to the UK Government on medical matters, with particular responsibilities regarding Public Health. From 2004-2016, Dame Sally was the Chief Scientific Adviser for the Department of Health, where she was actively involved in NHS R&D from its establishment and founded the NIHR. Dame Sally received her DBE in 2009.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!