Drug Development/R&D
New medicines lead to improved patient outcomes and societal benefits, but R&D is a long and costly process. Ongoing health economics research aims to find the right balance between incentivising manufacturers to innovate and ensuring affordable access for health systems.

As UN General Assembly Highlights Tuberculosis Fight, Will BRICS Lead on the R&D Agenda?
26 September 2018
The Center for Global Development and the Office of Health Economics are working on a new approach to drive the next generation of investment for better…

OHE Lunchtime Seminar: The People’s Prescription – Re-imagining Health Innovation to Deliver Public Value
7 September 2018
OHE Lunchtime Seminar with Mariana Mazzucato on Re-imagining Health Innovation to Deliver Public Value. 28 September 2018 12:00-2:00pm.

Incentives, Competition, and Pharmaceutical Innovation in Europe: The Case of Direct Acting Antivirals for Hepatitis C
26 July 2018
To what extent can R&D incentives, competition and other factors facilitate access to highly valuable and costly pharmaceutical innovations?

How Much Should Society Pay for a New Orphan Drug? A Contribution to this Debate is now Available in a New OHE Research Paper
18 July 2018
What would be the price for an orphan drug that generate rates of return no greater/smaller than the industry average? Is it reasonable?
Seminar Briefing: The UK Biotech Sector and Brexit
1 May 2018
In this paper, Sir Geoffrey Owen and Dr Michael Hopkins discuss ‘The UK Biotech Sector and Brexit: Past Performance and Future Prospects’.

New Publications: Optimal Development and Use of Real-World Evidence for Coverage and Formulary Decisions
18 April 2018
Just published are two new reports that explore the use of real world evidence for coverage and formulary decisions. The publications represent 1) the background paper…
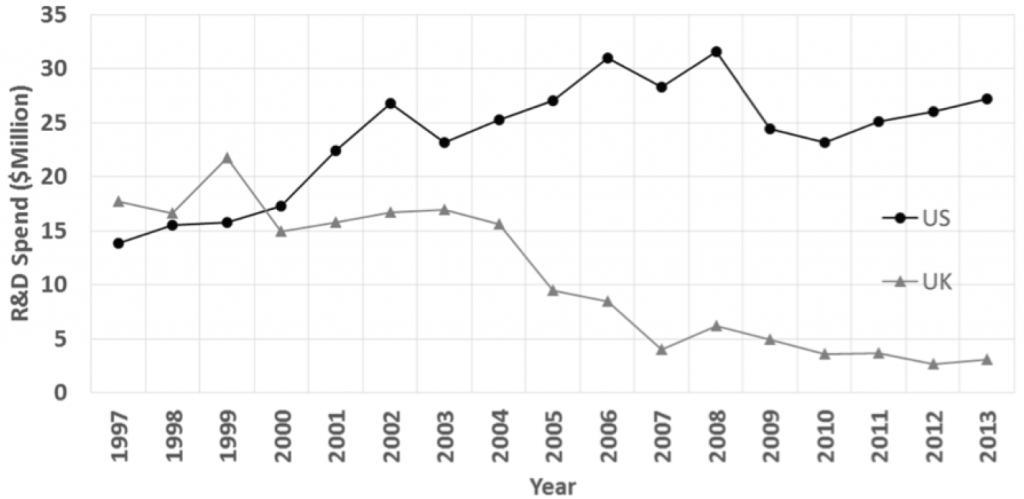
R&D, Competition and Diffusion of Innovation in the EU: The Case of Hepatitis C
1 July 2018
Using a multidisciplinary methodological approach combining a theoretical economic framework with uptake/market share analyses by country and interviews, this OHE research concludes that: (i) IP incentives…

Antimicrobials Resistance: A Call for Multi-disciplinary Action. How Can HTA Help? A Summary of a Symposium held at HTAi Rome 2017
31 October 2017
OHE has just published a new briefing entitled ‘Antimicrobials Resistance: A Call for Multi-disciplinary Action. How Can HTA Help?’

Opportunities for Utilising ‘Real World Data’ in South Korea
20 October 2017
OHE publishes a report assessing the capacity in South Korea for the collection and use of real-world data in health care.