Sign up to our newsletter Subscribe
Challenges and Solutions for Budget Impact Analysis of Gene Therapies

Shortages have been observed across a range of therapeutic areas, particularly in the off-patent generic and biosimilar markets. Optimal policy design and decision-making require an understanding of the root causes of drug shortages, as well as their impact.
Very low prices in some generic and biosimilar markets are a key driver of drug shortages.
In previous research, we demonstrated that price erosion – caused mostly by cost-containment policies – is a key underlying driver of drug shortages. Commercially unattractive markets with low profit margins result in markets with few producers. This limits the bandwidth and resilience to deal with demand shocks or supply issues, resulting in a higher likelihood of a drug shortage occurring. Healthy generic/biosimilar markets should therefore be a key focus for policies addressing drug shortages, which may mean easing cost-containment policies in order to promote better investment in more resilient supply chains. This could mean higher prices and spending in the short-term, to reduce the risk and costly impact of drug shortages in the longer term.
For decision-makers to understand this trade-off, it is important to know more about the cost of drug shortages: their potential scale, how they manifest, and who bears those costs. This can then promote better-informed decisions around the design and scale of policies to mitigate drug shortages.
The disruptions caused by drug shortages impact health systems and patients via three key routes: price increases, additional healthcare resource use, and patient health impact.
To design a framework to estimate the cost of a drug shortage, we examined the literature over the last 10 years to identify the key themes and contributing elements to the impact and cost of drug shortages. Price increases were identified to be a key feature of drug shortage impact, encompassing both the cost of switching to higher-cost alternatives, as well as an upward trend in the price of the drug itself in shortage. Additional healthcare resource use captures the time and labour costs associated with managing the shortage, particularly for pharmacists, e.g. adjusting inventories, identifying alternatives and substitution protocols, and altering purchasing practices. Finally, the patient’s health and quality of life impact must be considered as the most serious risk and consequence of a drug shortage, in cases where the shortage leads to delays in treatment or sub-optimal care.
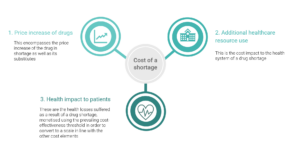
Framework for estimating the cost of drug shortage
We combine these three factors to generate a framework for estimating the cost of a drug shortage, which we then applied to three hypothetical case studies selected to represent a breadth of drug shortage impact scenarios across treatments with very different characteristics (biosimilar, small molecule generic, generic injectable), applications, patient populations, and market dynamics: trastuzumab, statins and saline.
The potential cost impact of drug shortages is huge. Relative stakeholder impact varies greatly depending on the type of drug in shortage. Effects differ by setting, with marked differences between the US and Europe.
Trastuzumab is a highly effective targeted biological therapy for the treatment of early-stage HER2-positive breast cancer. No large-scale shortages of trastuzumab have yet been reported. We found that the cost to the health system and patients of a trastuzumab shortage could be $519 million in the US and €16.6 million in the EU. The greatest impact of a shortage of trastuzumab is likely to be the health losses suffered by patients who either are denied or face a delay in access to treatment. The likely scale of the health system and patient disruption, as well as subsequent costs, is highly sensitive to the proportion of patients that we (assume) cannot switch treatments because of the limited total supply of trastuzumab resulting from the shortage.
Statins are a widely prescribed small-molecule therapy used to lower cholesterol and reduce the risk of heart and blood vessel disease. No large-scale shortages of statins have yet been reported. We predict that the cost of a statin shortage would be driven by additional healthcare resource use (EU) and price increases (US). Although patient disruption is likely to be small (given many alternative products that patients can switch to), the health system costs of managing a shortage and switching patients to alternative therapies are likely to be significant given the huge scale of statin use. For this reason, healthcare resource use represented the biggest proportion of costs for the EU. In the US, the cost of purchasing alternative statins is likely to be significant, particularly considering price increases that could occur. Total costs using our base case assumptions reached $1.45 billion in the US and €63 million in the EU. The large difference is explained by the inclusion (for US) or not (for EU) of costs associated with price increases of the drug in shortage and its alternatives. This is because for the EU case, we assume that the cost of purchasing substitute drugs is covered by the manufacturer.
Saline has multiple uses and is a key medical supply for hospital-based care. Disruption in saline production has become more commonplace over recent years. The market concentration of saline production is high, meaning that there is a high risk that any disruption will lead to a critical supply shortage, causing prices to rise and hospitals to ration. We anticipate the impact of a saline shortage to have the greatest impact on staff time and the health care system. The health system and patient costs of a saline shortage are very difficult to quantify given the broad use of saline across all elements of hospital care but are likely to be extremely high.
Policymakers should weigh shortage costs against expenditure control benefits to foster sustainable drug markets and resilient supply chains.
The literature review and illustrative case studies highlight the broad spectrum and heterogeneity of drug shortage impacts, in terms of the stakeholders they most impact, the degree of impact, and how they differ across settings. They also demonstrate the potentially huge magnitude of those costs.
While precise estimates are impossible, and results are highly sensitive to changes in the assumptions made, there is no doubt of the critical impact of drug shortages on patients and health systems. Although databases tracking the number of shortages exist, further research is needed to track their impact. We contribute a general framework for the cost estimation of a drug shortage, applied to some hypothetical case studies. These estimates need to be refined and generalised with further research.
We find that the costs to society of key medicinal products running into shortage could be huge. Existing policies that emphasize cost-containment and expenditure control, particularly for off-patent medicines, overlook the risks of drug shortages that those policies may cause, and their potential catastrophic costs. Policymakers should consider this trade-off, in order to promote sustainable drug markets and resilient supply chains in the long-term.
This report, ‘The Cost of Drug Shortages’ was commissioned and funded by Organon Pharma (UK) Limited.
The Cost of Drug Shortages
