Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

This report examines the likely success of the newly proposed pull incentive for antibiotic development in the UK by seeking the perspectives of (potential) investors in the antibiotics space. Investors are key stakeholders in the development of new antibiotics, as their buy-in is critical to unlock the required funds and expertise for the development of novel antibiotics.
Nine interviews were conducted, involving financial investors, and biopharma executives. We found that most investors were generally optimistic that a pull incentive could be a useful policy tool if it provides a sufficient monetary incentive globally (which will require high levels of consistency on eligibility and award/scoring criteria between jurisdictions) and is sufficiently predictable for investors and innovators.
While the UK effort in isolation will not represent an effective pull incentive, combined action from the EU and the USA would be expected to provide a sufficient minimum pull incentive. Substantial harmonisation on the target products supported across schemes will be required for global sums to add up to a sufficient incentive, see Figure 1.
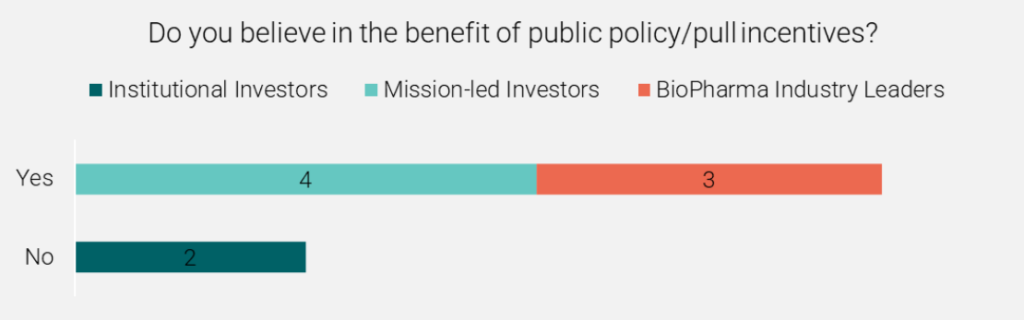
Figure 1 Investor perspective on public policy/pull incentives in AMR
The results of the interviews with investors indicate that there are several areas in which current UK proposals could be strengthened, enabling them to become a more convincing and effective policy tool for investors:
The report was commissioned and funded by The Association of the British Pharmaceutical Industry (ABPI).
Proposals for a Novel UK Antimicrobial Subscription Model: The Investor Perspective

