Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe


Just published are the results of an OHE Lunchtime Seminar given by Prof Riccaboni, from the University of Trento. In this publication, he explores whether R&D productivity in the pharmaceutical industry has declined and, if so, why. Just published are…
Just published are the results of an OHE Lunchtime Seminar given by Prof Riccaboni, from the University of Trento. In this publication, he explores whether R&D productivity in the pharmaceutical industry has declined and, if so, why.
Just published are the results of an OHE Lunchtime Seminar given by Prof Riccaboni, from the University of Trento. In this publication, he explores whether R&D productivity in the pharmaceutical industry has declined and, if so, why[1].
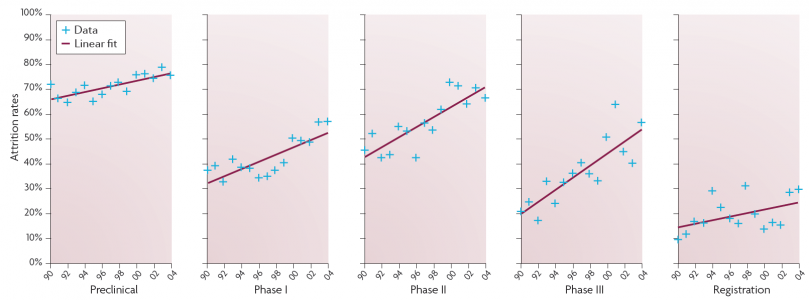
Launches of new products, Prof Riccaboni notes, have remained at about the same number per year since 2000 and have remained at a level lower than during the 1990s. He identifies two reasons for this. First, development times (patent filing to market launch) have increased markedly. From an average of 9.7 years in the 1990s, development times increased to an average of 13.9 years after 2000. Second, “attrition rates”, i.e. the percentage of R&D projects that are discontinued, have increased – and particularly in Phases 2 and 3.

Trends in attrition rates of drug development projects
Several reasons for these trends are identified by Prof Riccaboni:
In this Seminar Briefing, Prof Riccaboni tracks the shift in the composition of R&D portfolios towards more complex and higher risk areas of research. For example, comparing the R&D portfolio composition from 1990-1999 to that of 2000-2007 shows “an 8% increase in cancer research projects and a 4.57% decrease in the cardiovascular area.” Also increasing is research in anti-neoplastic agents (6.9%), neurological diseases (1.1%), and alimentary tract metabolism (1.6%). R&D in all other areas has been declining. The reasons for the shifts, according to Prof Riccaboni, are a mix of company strategy, i.e. deciding that higher potential rewards are worth higher risk, and public incentives, e.g. allowing only “breakthrough” drugs to be eligible for higher pricing.
In comparing R&D performance of companies based in the US and Europe, Prof Riccaboni also identifies a set of “global” companies that seek out new opportunities and talented people outside the countries in which they are headquartered. He finds that, overall, the R&D productivity of these global companies is highest. With respect to sales, “the market value of NMEs for European companies is far lower than the market value for US companies even after controlling for a number of variables”, he notes, but he found “no difference between the market value of drugs produced by US companies and that of global companies that do most of their research in the US.”
Download Riccaboni, M. (2012) Is there a productivity crisis in pharmaceutical R&D? Seminar Briefing. 11. London: Office of Health Economics.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!

