Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe


This second blog focuses on the value assessment used within the NICE-NHS England AMR pilot, which is crucial for capturing an antibiotic’s value so the subscription model can reward those with higher value.

This article was commissioned by Pfizer Inc. Content was written by OHE Consulting Ltd with technical review from Pfizer Inc.
The first blog in this series on the economics of antibiotics explained why markets fail to incentivise the development of novel antibiotics. It also outlined how England has spearheaded efforts to test a potential solution to this challenge through a subscription model for antibiotics in the NICE-NHS England AMR pilot. This second blog focuses on the value assessment used within the NICE-NHS England AMR pilot, which is crucial for capturing an antibiotic’s value so the subscription model can reward those with higher value.
Trying to change the rules of the game
The Health Technology Assessment (HTA) model has been used in England for decades to assess the value of medicines and decide whether or not the NHS will pay for them [1]. The first problem with the reimbursement of antibiotics is that HTA, as it is traditionally carried out, uses methods that are inappropriate for antibiotics leading to their undervaluation, subsequent low prices and weak incentives for developers [2]. The second problem is that a payment mechanism by the NHS based on volume-linked reimbursement can compromise existing and future antibiotic stewardship- that aims to limit inappropriate use of antibiotics and therefore, in some way; limit the volumes of antibiotics used [3].
There are various methodological shortcomings of traditional HTA when applied to antibiotics, such as the choice of a single comparator instead of the more complex reality in which a diversity of products is used; the use of health-economic models don’t fully capture transmission effects or the impact of growing resistance over time; and the focus on narrow benefits to patients treated with a specific antibiotic while ignoring several important wider population effects, prevent to capture the broader value of antibiotics to society appropriately [4].
In recognition of the shortcomings of traditional HTA, the NICE-NHS England AMR pilot aimed to improve on the status quo with a new HTA method specifically designed to capture such broader value of antibiotics [5]. By combining this novel HTA approach with the subscription-based payment model it is possible to create a procurement mechanism that is in line with good antibiotic stewardship [3].
Ready, STEDI, Go
The AMR model’s HTA process is based on a novel value assessment framework for antibiotics proposed by Rothery et al. [6] following work by OHE [4]. One of its most apparent differences compared to the status quo is the introduction of five novel (‘STEDI’) value elements (a term adopted by Outterson and Rex [7]) to capture the broader value of antibiotics summarised in the image below adapted from the definitions used by Karlsberg-Schaffer [4] and Rothery et al.[6].
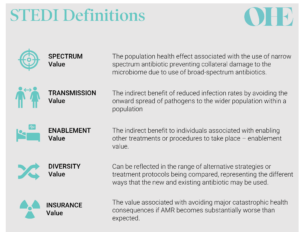
The introduction of the STEDI elements is a game-changer. Similar concepts have been proposed for other health technologies, such as vaccines or advanced medicinal therapeutical products like gene therapies. But STEDI has become the first broader value framework that has been agreed by payers and HTA bodies and implemented in practice.
Why STEDI is challenging to implement
Considering these broader value elements is necessary to capture the value of antibiotics but the pilot showed that measuring each value element appropriately is challenging.

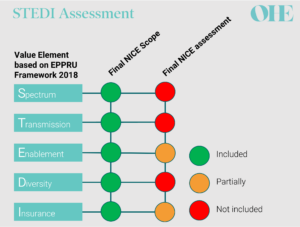
EEPRU recommendations compared to NICE final assessment for both products included in the pilot [8-11]
All parties agreed that neither of the antibiotics in the pilot produced spectrum value because they were both broad-spectrum antibiotics [8,9]. However, opinions differed on the other STEDI elements. The health economic models used by NICE’s commissioned modelling team captured only two of the other STEDI value elements – and even then, only partially. The stated reasons for this included large structural uncertainty (e.g. related to structural modelling choices) and/or lack of relevant evidence contributing to parameter uncertainty [8,9].
Importantly, NICE’s decision-making committee substantially adjusted the valuation for both products to account for missing enablement, diversity and insurance values. The committee also adjusted the valuation because it considered that both antibiotics serve a larger population than was modelled. These two adjustments increased the valuation by 20% and 50% for each of the antibiotics included in the pilot [8,9].
The value of both products after the adjustments by the NICE committee exceeded the capped annual payment set out by the NHS (which will be explained in detail in the next blog). However, the valuation may well have underestimated the true value of both products due the uncertainty in defining the right population size and in capturing the full STEDI value [8,9].
What’s next for the value assessment of antibiotics?
The pilot exposed the opportunities and challenges that lay ahead for the value assessment of antibiotics. All stakeholders should focus on improving the underlying value assessment methodologies. A value assessment process that requires the NICE committee to guesstimate important value elements is not a sustainable approach. Any future value assessments using STEDI must ensure that the underlying modelling provides credible value estimates, for example, by using dynamic transmission models to capture transmission effects, such as is done for vaccines.
Even with the best model in the world, the valuation would still have been limited by a lack of data: a challenge the NICE committee also recognised in its recommendations for further research. Importantly, it recommended improving data collection on the relationship between usage scenarios for antibiotics and the rate of emergence of resistance [8,9]. The emergence of resistance over time is a crucial component underlying all the STEDI elements and is likely to require significant investment into methods and infrastructure to measure [8,9].
There’s also room for improvement in the process before any evidence is assessed. Pfizer developed and submitted their own model for their product that covered both the traditional (patient and health system) value and attempted to capture larger parts of STEDI elements. However, NICE relied almost entirely on the model commissioned from the Policy Research Unit in Economic Evaluation of Health and Care Interventions (EEPRU) for its conclusions [8,9]. Since modelling the value of an antibiotic over time is complex and resource-intensive, such redundancy should be avoided. It might be preferable to shift the process towards something closer to a technology appraisal, where the company submits the model which is then reviewed by an External Assessment Centre with appropriate expertise. In doing so, NICE could focus on developing suitable methods for modelling the STEDI elements, choosing alternative antibiotic use scenarios, and estimating health effects from microbiological data, so setting the rules which will guide the ’company’s submission.
The elephant in the room: will it even work?
What is clear is that HTA for antibiotics is still developing. With a novel model to assess and pay for new antibiotics England took an essential first step. Even if we manage to overcome all of the uncertainties with the new HTA model, there is still the question of whether the delinked subscription model will be enough to stimulate the global pharmaceutical market? We consider this question in the next blog in our series.
The Economics of Antibiotics blog series
In this four-part series, we discuss the economics of antimicrobial resistance to understand the relevance of the NICE-NHS England AMR model and explore the questions that remain about the delinked model:
References
PP-UNP-GLB-0506; September 2022
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



