Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe


US policy makers are discussing an attempt to lower prescription drug spending in the US. US policy makers are currently discussing the Elijah E. Cummings Lower Drug Costs Now Act (commonly referred to as H.R. 3), an attempt by the…
US policy makers are discussing an attempt to lower prescription drug spending in the US.
US policy makers are currently discussing the Elijah E. Cummings Lower Drug Costs Now Act (commonly referred to as H.R. 3), an attempt by the Biden administration to lower prescription drug spending in the US. Over the last several weeks, OHE engaged academic economists, venture capitalists and other investors in life sciences, key decision-makers at biopharmaceutical companies, and other experts to gather perspectives on H.R. 3 and its impact on future R&D investment. We will summarize these findings in our 5-part blog series on H.R. 3. In this first instalment, we discuss why experts in our recent study felt H.R. 3 was the wrong policy for the wrong problem.
As currently written, H.R. 3 would allow the government to cap the prices of certain high-expenditure prescription drugs chosen by the Secretary of Health and Human Services. Although characterized as negotiation, manufacturers who do not comply with government-set prices can be charged an excise tax of up to 95%. These prices will be made available to Medicare Part D and commercial insurers, thereby potentially reducing prices – and revenue – across the whole US market.
Initial analysis by the Congressional Budget Office (CBO) estimates that the H.R. 3 price-setting provisions would reduce direct federal spending by $456 billion over the period 2020-2029 and all eight provisions taken together would reduce unified federal deficits by $5 billion over this period. Despite the estimated savings experts in our study believed that drug pricing policies such as H.R. 3 would not address the systemic problems with the US healthcare system.
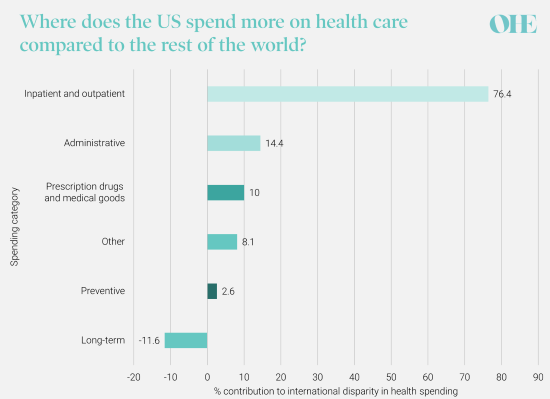
The US is recognised as providing rapid access for many citizens to the latest innovative medicines and technologies. Nearly 90% of all newly launched medicines are available in the US while only about half are available in other countries such as Canada and France. The US spends more on health care than any other developed country – 17.1% of US GDP ($3.8 trillion) in 2019 and double the per capita spend in Canada or Australia – driven largely by higher spending on inpatient and outpatient hospital care as the graph below illustrates.

The distribution of difference in per capita health spending between the US and comparable countries, by spending category, 2018. Original graph and data: How does health spending in the U.S. compare to other countries? – Peterson-KFF Health System Tracker
However, US life expectancy lags substantially behind that of other developed nations. While this may be partly explained by poor access to health insurance and inequality in access, even those with insurance may face high out-of-pocket costs for essential medicines.
H.R. 3 aims to reduce government spending on prescription drugs but does not address the patient affordability problem in US healthcare. Only a small proportion of the international disparity in per capita healthcare spending is attributable to higher spending on prescription drugs and medical goods. And a large factor driving the high cost of medicines may also be attributable to markups by intermediaries such as wholesalers and hospitals rather than pharmaceutical companies themselves, who are the sole target of H.R. 3.
In our study, the majority of experts surveyed judged that H.R. 3 was unlikely to materially improve affordability. This is because it focusses on only one major medical input (pharmaceuticals) without addressing any of the systemic flaws that distort the prices of health-related goods and services, and inflate out-of-pocket spending for patients.
Critically, policies such as H.R. 3 can cause significant unintended consequences. Proponents commonly ignore the likely strategic responses of pharmaceutical companies targeted by H.R. 3. The policy relies on reference pricing, which itself is dependent on price data being available in foreign reference countries. However, firms could avoid or delay launching their new drugs internationally to avoid creating a lower reference price, thereby limiting or eliminating access for foreign patients. Insights from our expert interviews suggest that such responses are highly likely.
Interest in H.R.3. has been reignited lately as part of the reconciliation bill to pay for Biden’s $3.5 trillion infrastructure and fiscal reforms, and given expenditure on pharmaceuticals under Medicare Part D is over $180 billion a year this may initially sound attractive. A focus on poorly targeted short-term savings could have major adverse consequences for patients in the future by causing an immediate decline in R&D spending feeding through to fewer new drugs coming onto the market in the next few decades.
These stark unintended consequences of H.R. 3 arise partly because of the high social value of spill-overs from inherently risky pharmaceutical innovation. Spending on today’s cancer therapy supports R&D for tomorrow’s cure for Alzheimer’s disease. Also, it was the “re-tooling” of pre-existing mRNA platforms that allowed for the rapid development of COVID-19 vaccines. These positive externalities must be considered when evaluating a policy such as H.R. 3, which could have a dramatic impact on R&D spending in the pharmaceutical and biotechnology industries.
The experts consulted in our study agreed that drug price controls would have a dramatic impact on biopharmaceutical revenues, reducing investment in R&D and resulting in fewer critical medicines for patients. This finding strikes a chord with a 2018 report by the National Academies of Sciences, Engineering, and Medicine which emphasized that: “In the end, drugs that are not affordable are of little value and drugs that do not exist are of no value.” What the US needs to devote resources to is designing and implementing a better way to link financial rewards for drug innovators to the value that US patients place on those innovations.

In the next OHE H.R. 3 Series blog, we will discuss how our interviewees suggested that economic impact evaluation of H.R. 3 undertaken by the Congressional Budget Office (CBO) rests on an overly simplistic view of how biopharmaceutical investors grapple with the risk-return trade-off given a rapidly expanding scientific knowledge base. The CBO modelling is based on historical correlations of unknown relevance to current trends, and thus provides a weak evidence base for major policy changes.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



