Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe


In this Insights series, Around the World in HTAs, we shed light on HTA around the world. In this edition, Patricia Cubi-Molla & Jake Hitch, OHE, and Róbert Babeľa, Slovak Medical University, take us to the Slovak Republic.

The healthcare system in the Slovak Republic
The Slovak Republic has universal basic health coverage based on a compulsory health insurance scheme. Health insurance companies (HICs) contract directly with healthcare providers and most Slovak citizens (63–65%) are insured by the public state-owned HIC.
Health technology assessment (HTA) has only been required as part of applications for public health insurance reimbursement since 2011. From 2011 to 2022, an arm of the Ministry of Health (MoH) was responsible for reviewing HTA and pharmacoeconomic analysis submissions for innovative medicines. An independent national HTA agency – the National Institute for Value and Technologies in Healthcare (NIHO) – was set up in 2022.
NIHO uses EUnetHTA methods to provide information for reimbursement decisions made by the MoH regarding all innovative technologies that have a budget impact of over €1.5 million per year. Innovative technologies with a smaller budget impact are evaluated by internal HTA specialists within the Drug Policy arm of the MoH.
A roadmap for new technologies
The current reimbursement process is similar for medicines, medical devices, and dietary foods. Figure 1 illustrates the process for outpatient drugs. The pharmaceutical company first obtains marketing authorization from either the European Medicines Agency (EMA) or the Slovak Republic State Institute for Drug Control (SIDC). Conditional on successful marketing authorization, the drug developer then applies to the MoH for reimbursement.
The Reimbursement Committee advises the MoH on reimbursement decisions, informed by input from the expert working groups, NIHO, and the Section of Pharmacy and Drug Policy. Expert working groups provide clinical assessments, NIHO provides technology assessments, and The Section of Pharmacy and Drug Policy issues opinions and evaluations. The Reimbursement Committee makes a recommendation to the MoH who has the final say on whether the technology should be fully or partially covered by HICs.
The process should take 180 days from submission to decision for a new medicine and 270 days for medicines that are already reimbursed but require a new assessment process (for example, those submitted for a new indication). There is an appeals process that involves the Reimbursement Council and the MoH. See here for more details.
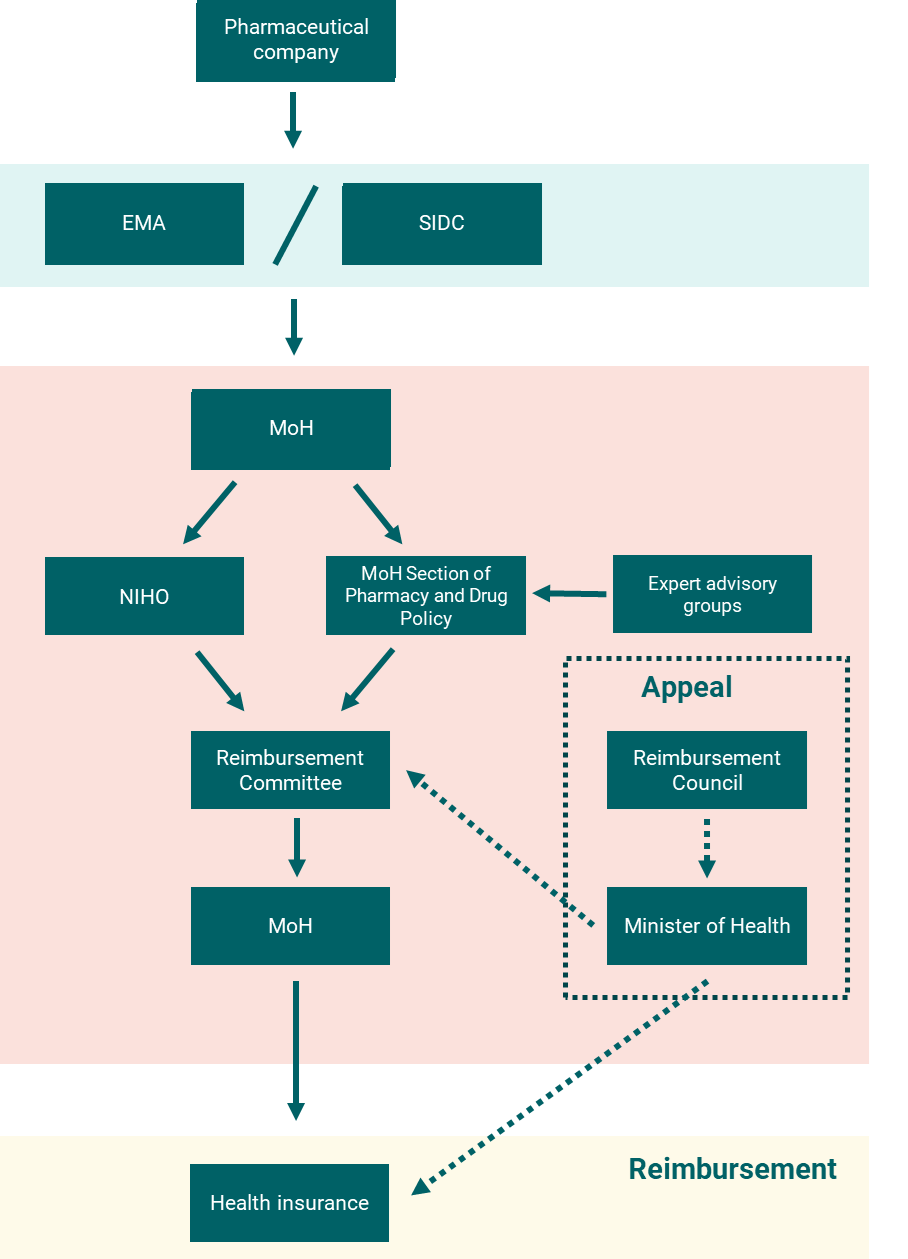
Figure 1: Overview of the outpatient drug reimbursement process in the Slovak Republic
Current challenges for HTA of medicines
The history of HTA in the Slovak Republic is relatively short and therefore there are many open challenges. These include choosing appropriate methods and parameters for the economic evaluation of new health technologies, as well as practical issues such as shortages of HTA professionals. In this blog we focus on the surprisingly impactful issue of discounting, drawing on recent OHE research to update the discount rate for the Slovak Republic.
People generally care more about current outcomes than future outcomes. In economic evaluations, this time preference is reflected through a technique called discounting. While it may seem like a technical exercise, discounting can have significant effects on the results of cost-effectiveness analyses (CEA), particularly for interventions that have high upfront costs and benefits that accumulate gradually over time, such as gene therapies for inherited retinal disease. To the extent that CEA is an important element in HTA, discounting can have significant effects on final coverage and reimbursement decisions. It is therefore imperative that the right discount rate(s) is used.
The discount rate currently required for health-related costs and benefits in pharmacoeconomic analysis in the Slovak Republic is 5% per year. Most other countries use a lower discount rate (Figure 2), including the Slovak Republic’s neighbours, the Czech Republic (3%) and Hungary (3.7%). Partly on this basis, a main challenge for decision-makers in the Slovak Republic is to reconsider how they weigh up costs and health today versus tomorrow.
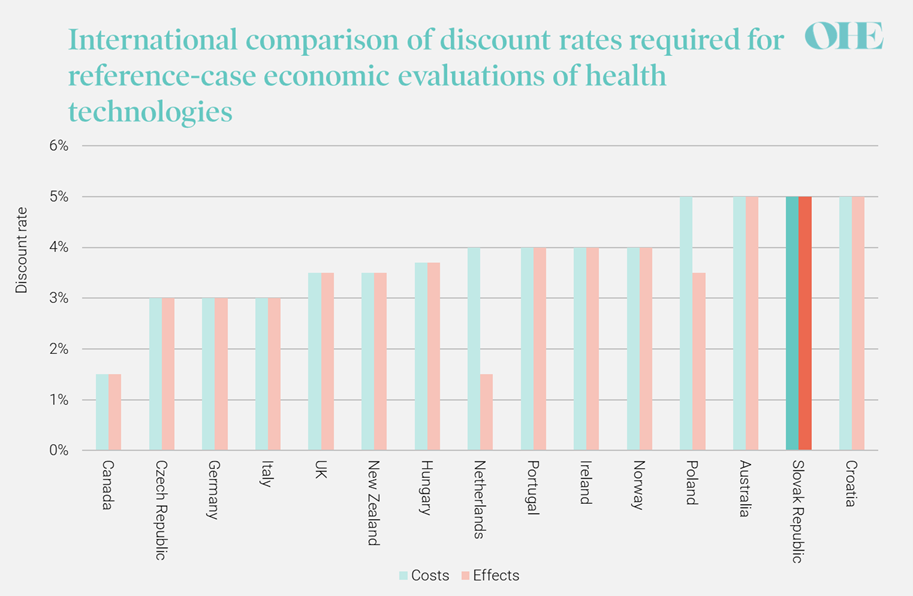
Figure 2: International comparison of discount rates for reference-case economic evaluations
Next steps for HTA in the Slovak Republic
Based on recent macroeconomic and demographic data, OHE research recommends a discount rate of 3.3% based on its best estimate of the Slovak Republic’s social discount rate. This estimate is significantly lower than the most recent official estimate used to validate a discount rate of 5% because the OHE estimate for expected consumption growth – a key input in the calculation – is significantly lower than the official estimate. The official estimate is based on historic consumption growth, which has been argued to be a misleading indicator of future growth for former transition economies such as the Slovak Republic.
A key next step would be the publication of an official HTA guidelines which outline the HTA process and methods for pharmacoeconomic analyses. These guidelines should include the principles for incorporating the analyses performed by the newly formed NIHO into official coverage and reimbursement decisions. As part of the official HTA guideline, we advocate for an increasing transparency around the choice of discounting method and rate(s) for use in HTA.
Investment to increase the number of trained HTA professionals in the country is another key priority. This could be achieved through an expanded budget for academic HTA programs and PhD research, and national policies to attract experienced HTA professionals from other countries.
Since 2021, there has been substantial progress in enhancing and standardizing H TA in the Slovak Republic. However, there is still a considerable amount of work to be done. Methods for pharmacoeconomic analysis and shortages of HTA professionals are two key challenge areas. Decision-makers should also start to consider a broader range of information in HTA beyond narrowly defined patient health and healthcare system costs, including productivity costs and disease burden, in order to assess the comprehensive impact on society. Progress on these issues is needed to ensure decision-makers consistently make the right coverage and reimbursement decisions for the country.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



