Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe



Today marks Rare Disease Day 2025. This time last year we published an Article entitled “Why do we care about rare?”, summarising the origins of rare disease day, the fundamental ambition – to strive for more equitable access to diagnosis, treatment and care for rare disease patients– and the breadth and depth of OHE research on the topic over the past several decades.
In this article, which is jointly authored by members of OHE’s Economics of Innovation research team, we want to show you why we still care about rare, and to demonstrate our continued commitment to current issues impacting rare diseases. We outline priority topics and challenges that urgently need to be overcome, to: (1) enhance our understanding of rare diseases; (2) improve incentives and access to innovation, and (3) create an enabling policy environment for advances in care.
It all starts with patient data
There are around 7,000 (known) rare conditions, 80% of which have a genetic cause. The definition of “rare” means that a disease impacts fewer than one in 2,000 people, but the majority of rare diseases – around 85% – affect fewer than one in a million.
Genomic sequencing techniques, which involve decoding a human’s DNA, have revolutionised rare disease diagnosis. However, significant hurdles remain and the “diagnostic odyssey” is all too common, with an average time to diagnosis of around 5 years. Health systems must strive for better. An accurate diagnosis unlocks the ability for rare disease patients to access existing support, and to both learn from – and contribute to – the evolving data and evidence landscape for a condition.
What do we mean by data? Data is information collected through observation or experimentation. It may be accumulated through clinical trials, routine clinical appointments, electronic health records, disease registries, patient observations, or notes scratched diligently by parents into their baby’s red book. It includes any information that can help to build a better picture of the condition and the way it impacts patients,
The collection of data underpins evidence generation. For rare diseases in particular, we need to collect and compile more of it. Yet, data capture is – by definition – limited by the small patient numbers impacted by any individual condition. Global efforts to invest in and improve data collection infrastructure and data linkage continue, which is essential for rare conditions that of course do not recognise country borders.
But there are huge challenges. In England, improving the “findability” and data captured on people living with rare disease has been specifically targeted as a core action in successive Rare Disease Action Plans. The National Congenital Anomaly and Rare Disease Registration Service (NCARDRS), which is supposed to deliver on this action, captures about 1800 rare conditions – accounting for only around a quarter of known rare diseases.
About 95% of rare conditions lack an approved treatment. But capturing and tracking patient data, even when no treatments yet exist, is crucial to improving our scientific understanding of a condition and building a picture of “natural history”, which in turn can help identify biomarkers, accelerate drug development, and provide the evidence to support the evaluation and access to the treatments of the future.
Patient access to innovation
Policy levers to improve incentives for innovation in rare disease are critical. These can include regulatory incentives (such as extra market exclusivity) or reimbursement incentives (e.g. rewarding drugs that address rare conditions and high unmet need with higher prices). In our Insight Article published on Rare Disease Day 2024, we described some of those levers in detail.
A recent publication by CIRS identified high levels of regulatory flexibility in the approval of orphan medicines across jurisdictions, leading to an increase in orphan drug approvals between 2018 and 2022. However, they observe that these conditions are not extended to decisions at the reimbursement stage, and call for greater alignment between regulatory and HTA decision-making. This is particularly relevant for rare disease treatments that offer care advances in areas of very high unmet need, for which regulatory approval is often expedited but for which HTA bodies struggle to make evidence-based decisions in the presence high uncertainty.
Innovative pricing and payment models can help address the ensuing challenges around affordability or value uncertainty, de-risking the reimbursement decision. This topic is of particular interest to us at OHE, and forms the focus of our research as part of the EU-funded HI-PRIX project. We are conducting interview-based research with a range of stakeholders, finding that – under the right conditions – innovative payments models can accelerate patient access while ensuring affordability to payers and incentives to innovators. Examples include the outcomes-based staged payment model used in Italy and Spain for CAR-T cell therapies, as well as the “coverage with evidence development” approach adopted by NICE in the UK recently to support access to an innovative gene therapy for sickle cell disease (approved through the Innovative Medicines Fund).
Of relevance to ultra-rare diseases in the UK, Highly Specialised Technologies (HST) guidance provides a route to appraisal and access for some orphan medicines, with greater flexibility in the evidence considered and a higher cost-effectiveness threshold applied. NICE recently issued a consultation on amendments to the routing criteria for HST appraisal. While the proposed update makes some of the routing criteria more explicit, eligibility continues to apply to only a narrow subset of rare disease treatments. The graph below demonstrates that – for orphan drugs routed to the standard single technology appraisal (STA) – recommendation rates are lower than those routed to HST (71% versus 97%), which potentially relates to the lower threshold applied and the greater room for qualitative and multi-stakeholder input offered by HST. Over the last 10 years, timelines to a STA recommendation have been slightly longer for orphan compared with non-orphan drugs (median time between EMA approval and NICE STA decision being 13 months for orphan and 10 months for non-orphan). The timeline to recommendation via HST typically takes longer (a median of 21 months from EMA approval), potentially due to routing decisions, collation and submission of evidence, and more complex processes.
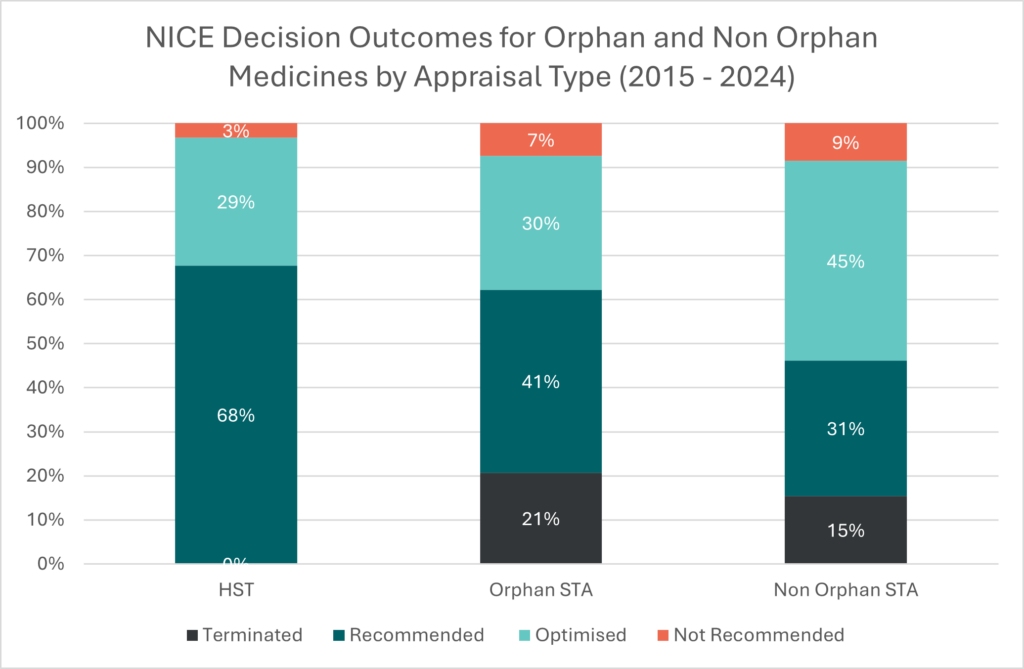
Source: Data published by NICE and collected and analysed by OHE. If you want to know more about HTA outcomes and timelines research, please contact Nadine Henderson: nhenderson@ohe.org
Fundamental to patient access to approved therapies is also a stable and resilient supply chain. In a recently published OHE report, we investigate the importance of diversity of supply in rare diseases markets. We found that enhancing supplier diversity strengthens healthcare resilience and offers treatment alternatives to patients, thereby improving patient access to innovation and health outcomes.
Policy environment
The policy environment for rare disease and orphan medicines is complex and encompasses regulation, reimbursement, and pharmaceutical market design. The challenge is to strike a balance between creating strong incentives for innovation and maintaining the sustainability and resilience of healthcare systems.
The EU Pharmaceutical Legislation is currently under review. The first proposal by the European Commission advocated for a curtailing of existing incentives, as reported in an OHE Insight blog. This is currently being debated with European Parliament and the European Council, but the uncertainty decreases predictability for orphan drug developers, posing particular challenges for small and mid-sized innovative biopharmaceutical companies, which account for a significant share of orphan drug companies. We will continue to keep you informed about developments in upcoming OHE Insight posts.
Another recent policy shift within Europe is the introduction of Joint Clinical Assessment (JCA), which has the potential to harmonise and speed-up access to treatments including orphans. Of critical importance to meeting this objective, however, is ensuring evidentiary standards applied to rare disease treatments are realistic and proportionate, and that the process is efficient, streamlining rather than delaying the route to local approvals and access. For example, this may require adopting flexible, orphan-specific evidence requirements to address inherent uncertainties in rare disease research. This could be informed by the experience of the Mechanism of Coordinated Access to orphan medicinal products (MoCA), a voluntary, non-binding, free-of-charge process that provides patients, payers and companies with the opportunity for early dialogue on the value of orphan medicines as outlined in previous OHE research.
Other significant policy developments include those we observe in the US, with the introduction of price negotiations as part of the Inflation Reduction Act (IRA). Notably, while drugs treating a single rare disease indication are exempt from negotiations, those treating more than one indication (an extremely common route for therapeutic advance for rare disease patients) are not. This could thereby disincentivise and reduce investment in follow-on indication research, limiting treatment options for rare disease patients. We conducted several interviews with investors in the pharmaceutical sector who confirmed that their investment behaviour has already been impacted. We aim to publish these interesting findings later this year. More recently, announcements by the new US administration around cuts to public funding of biomedical research are causing significant concern to rare disease communities in the US and beyond.
In a recent OHE report we highlighted some challenges and contradictions that can emerge in the rare disease policy space. We found that every country under study had an expressed goal of improving access to rare disease medicines, with most applying special considerations for orphan medicine reimbursement. However, rare disease medicines without orphan designations are typically not the beneficiaries of these considerations, making them less likely to receive positive HTA decisions. Similarly, we found that most of the countries studied relied on procurement strategies and HTA policies that can promote single-supplier markets, thereby reducing patient and physician choice and making these markets especially vulnerable to shortages. We recommend aligning policies at all levels—from national plans to HTA and pricing and reimbursement—to support a resilient healthcare and innovation ecosystem for rare diseases.
Promoting equitable access to medicines for rare diseases is a key component of the World Health Assembly’s Resolution on Rare Diseases, published on February 10th. In addition to urging member states to improve access to medicines – both broadly and through tools like innovative payment models – the resolution calls on member states to regularly assess the implementation of high-level rare disease frameworks and requests that the WHO publish a global action plan for rare diseases. These initiatives, if fully realized, could reconcile policies and improve access to medicines.
Conclusion
We have identified three key challenges for advancing the care of rare disease patients: strengthening research and data generation, enabling patient access to innovation, and designing strong and balanced policies to foster a sustainable innovation ecosystem for rare diseases. Health economic approaches can help address these challenges. OHE will indeed continue to care about rare in 2025 by contributing to research and the debate on solutions.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



