Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

This progress has been exemplified in the context of haemophilia, where iterative and incremental innovation has added significant value for patients and health systems. New treatments have transformed the lives of people living with haemophilia from a life expectancy of around 30 years in the 1960s to a life expectancy at present comparable to the general population in developed countries (Mannucci, 2020).
The impact of innovation on haemophilia
In haemophilia, continued investment in research and development has led to the development of a number of new innovations, each with unique benefits to patients. These include reduced treatment burden, improved adherence, and improved quality of life, including greater ability to partake in physical activities and to achieve major life goals. Further, new treatments provide patients with multiple options, meaning they can make treatment decisions based on their clinical needs, physical activity level, and lifestyle. These benefits also have spillover effects on carers, family members and wider society. Haemophilia thus provides an exemplary case study of how continued investment in pharmaceutical innovation can transform the lives of patients and carers.
Yet, despite an impressive array of treatments, there is a remaining unmet need that requires further innovation (Figure 1.). People with haemophilia still experience pain, joint damage, and mental burden due to their condition. Indeed, the aspiration for many people with haemophilia is to live with a haemophilia-free mind.
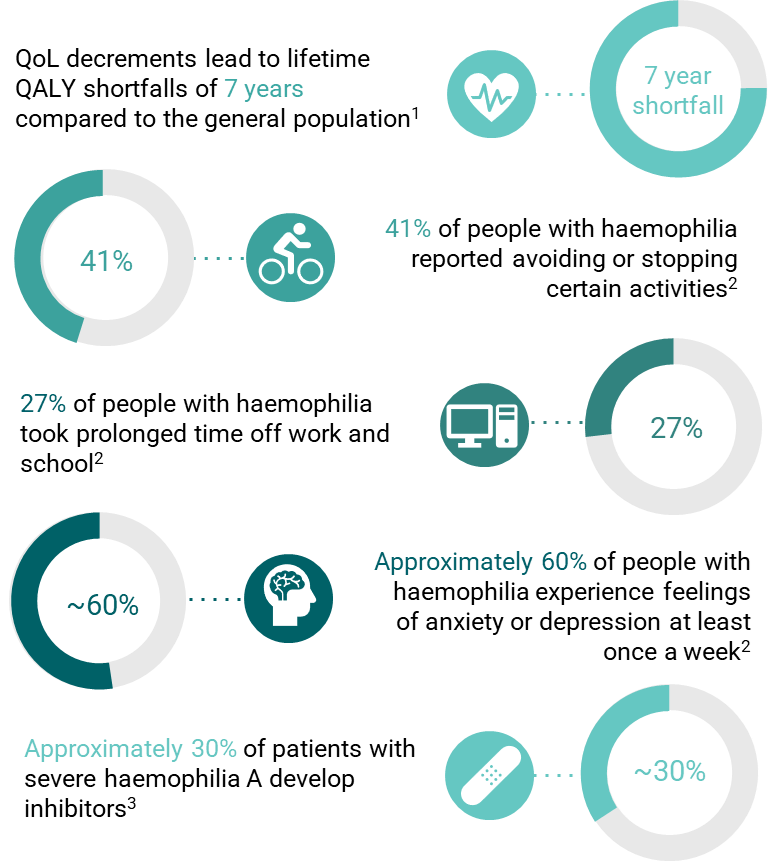
Figure 1 Unmet need in haemophilia
1Based on an ICER report estimating the expected years of life and lifetime QALYs associated with factor IX treatment, non-factor treatment and gene therapies for haemophilia A and B. When comparing lifetime QALYs to expected years of life, all treatments are associated with a QALY loss equivalent to at least 7 full years of life loss indicating a substantial unmet need due to morbidity (ICER, 2022)
2 Based on a 2023 global survey of people with haemophilia (The Harris Poll and Sanofi, 2023)
3 Approximately ~30% of patients with severe haemophilia A will develop inhibitors, in addition to 5% of patients with mild and moderate haemophilia A and 3% of patients with haemophilia B (Meeks and Batsuli, 2016).
Where next?
All stakeholders have a part to play in ensuring a healthy innovation ecosystem, allowing innovation to continue transforming patient lives.
Within haemophilia, pharmaceutical innovation has significantly improved care, but considerable unmet need remains. To further advance the research agenda in the haemophilia space:
‘Investing in Innovation: A Spotlight on Haemophilia Therapies’, was commissioned and funded by Pfizer.
A Spotlight on Haemophilia Therapies


Notifications