Sign up to our newsletter Subscribe
Challenges and Solutions for Budget Impact Analysis of Gene Therapies

Sign up to our newsletter Subscribe

OHE celebrates NICE’s 25 anniversary through a series of insights explaining NICE’s first steps and discussing NICE’s present and future role.

Introduction
Since its inception as an independent institution, NICE has not shied away from many challenges, such as the assessment of innovative interventions, the quality of evidence to inform decisions, and the demands to accelerate patient access to new technologies.
NICE’s response to these challenges is not exempt from criticism. In fact, the level of public scrutiny of NICE’s HTA methods updates has increased as NICE has amplified its international influence within the realm of HTA reforms. For example, the NICE HTA manual and its process of development were considered in Spain as a reference and in Australia as part of their ongoing review. All things considered, it is commendable that NICE has not only introduced method changes to keep pace with HTA developments and technology advances but has also increasingly published clear explanations of the rationale behind its decisions, reflecting a degree of transparency that is not always observed in other HTA agencies.
Building upon the insight discussing the creation and development of the NICE Technology Appraisal program, in this insight, we discuss how NICE’s approach has slowly evolved from reacting to changes in the landscape to anticipating upcoming challenges and seeking robust and pragmatic ways to address them.
Renew or die: keeping NICE HTA Methods guidelines fit for purpose
NICE published its first methods guidelines in 2001, replacing the interim guidance published in 1999 (Charlton, 2020). There have been four full reviews of NICE’s HTA methods guidelines, with updated documents published in 2004, 2009, 2013, and 2022. A new full review is not expected in the short run, but ‘modular updates’ will be performed (with the first modular update published in October 2023) to continue adjusting the manual to upcoming challenges.
The guideline reviews in 2004-2013 set key milestones, in particular, the explicit definition of a reference case and a cost-effectiveness threshold in 2004. However, the most recent full review of the NICE HTA manual on methods and processes was the most ambitious one. The review process started in 2019, and the discussion format and timelines had to adapt to the unprecedented situation of COVID-19. In addition, NICE needed to manage and review a high volume of material, including 56 proposals or ‘cases for change’. Nevertheless, NICE responded to the many demands for change by following a review process striking a balance between pragmatism and a good degree of stakeholder inclusivity. For example, most relevant documents were published, presenting arguments supporting each of the cases for change and the rationale behind NICE decisions. In addition, NICE ran two rounds of consultations to receive feedback from different stakeholders on the proposed changes. OHE responded to both consultations here and here.
The figure below summarises the main cases for change discussed during the review. In the traffic light figure, we painted red cases not incorporated in the new manual and those accepted in green. Further details about these cases can be found in an OHE insight series. The cases in yellow are a set of cases for change that were not accepted as further evidence, and research was needed to incorporate them into the manual.
Figure 1: Most relevant cases-for-change discussed for the most recent NICE Methods guideline review
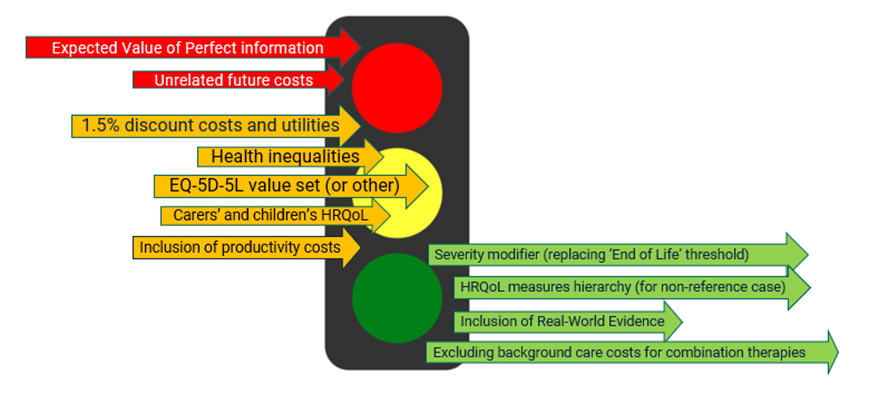
Unsolved challenges: ‘cases for change’ with a clear driver but still missing an enabler.
NICE does not recommend specific measures of health-related quality of life (HRQoL) in either children or carers as of yet, but it highlights the importance of further refinement of the methods and the need for additional research evidence. Similarly, NICE’s review supported the need to update the social tariffs used for the HRQoL measures, still supporting the collection of EQ-5D-5L data, but further research was needed before the new UK value set is ready for adoption.
Similarly, no change to the discount rate has been made. NICE committed to “collect evidence on the effect of discounting in future health technology evaluations, to help us contribute to further discussions on this issue“, but no further research has been identified in this space. Further work was also needed to introduce the consideration of health inequalities in health technology evaluations. NICE has done some research in the health inequalities space, but NICE’s position does not currently allow for a quantitative modifier for health inequalities (see for example, section 3.18 in a recent TA for treating sickle cell disease).
Proactive, not reactive: what is NICE’s agenda for the next 25 years?
NICE is slowly changing its approach from reacting to changes in the landscape to being more proactive, that is, anticipating upcoming challenges and looking for evidence-based and pragmatic ways to face them. The NICE strategy 2021-2026 illustrates this trend, indicating that NICE needs to “speed up [its] evaluation pathway and develop cutting-edge methods for emerging technologies that also consider their wider environmental and societal impact”.
Anticipated breakthroughs, such as new cell and gene therapies and digital health technologies, will drive further pressure to reform NICE processes and methods, leading to further reforms. Some will come from the last method review, such as the importance of incorporating health inequalities in a more explicit and maybe quantitative way and a move towards a societal perspective in value assessments. For other topics not covered in the last review, NICE is leading the international scene with new research and examples of applications in its decision-making. A clear example is the role of environmental impact in HTA and in healthcare systems (see NICE’s new guidance on the reduction in the use of desflurane, NICE’s decision aid on asthma inhalers, and a paper published, among others, by its Science, Evidence and Analytics Directorate).
Conclusion
NICE has demonstrated its adeptness in navigating a rapidly evolving environment while also exerting a significant influence within the realm of Health Technology Assessment (HTA), particularly in enhancing the transparency and predictability of its approach. While not all the cases for changes were advanced, and some criticism may arise regarding the perceived inadequacy of evidence as a reason, NICE’s decisions and associated research have yielded impactful evidence in other HTA systems. The primary challenge for NICE has been striking a balance between maintaining the quality of the HTA process and adopting a pragmatic approach, which allows for committee deliberations to play a pivotal role in decision-making. After 25 years of operation, it is evident that NICE has successfully met and overcome this challenge.

An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!



