Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe


A new OHE Consulting Report assesses information governance arrangements for real-world data in eight countries, and makes recommendations towards an ideal governance framework. Real world data (RWD) is a term that is becoming increasingly widespread in health economics and health…
A new OHE Consulting Report assesses information governance arrangements for real-world data in eight countries, and makes recommendations towards an ideal governance framework.
Real world data (RWD) is a term that is becoming increasingly widespread in health economics and health services research – with good reason. RWD is information that can be used for health care decision-making which is collected outside of an experimental clinical trial setting. RWD therefore has the benefit of reflecting outcomes in the “real-world”, i.e. routine clinical practice.
The environment for collecting data alongside clinical practice and using it to support decision-making by regulators and payers is changing. Regulatory flexibilities and increasing public pressure to facilitate earlier access to medicines (e.g. through adaptive pathways or early access schemes) means that regulators are increasingly monitoring benefits and risks throughout a medicine’s lifecycle; RWD is essential to support this.
Payers are similarly being challenged to conduct earlier value assessments under greater uncertainty, and re-visiting assessments as further RWD is collected. Moreover, managed entry agreements and arrangements for performance-based payments require data to be collected alongside clinical practice. These themes are central to the Accelerated Access Review currently being conducted by the UK’s Department of Health.
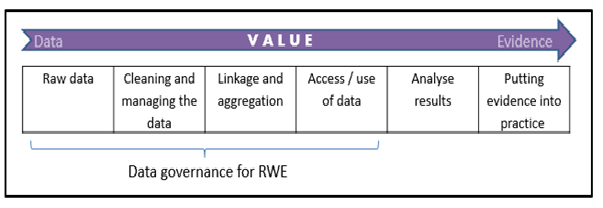
Supporting all of these developments is the improving infrastructure across the globe for data collection, for example through electronic health records. However, in order to utilise and derive value from RWD, appropriate and facilitative information governance is essential. RWD becomes real world evidence (RWE) – what is really of value to stakeholders – after a series of activities which facilitate the transformation of raw data into analysis and results. Robust and proportionate data governance, applied at each step, is essential in realising the value of RWD.
Figure 1: Value chain for generating Real World Evidence

OHE Consulting has just published a Consulting Report, commissioned by Lilly, which assesses the core governance arrangements for how RWD (both routinely-collected and de-novo) is accessed or generated, and used credibly to provide evidence in eight countries: the UK, France, Italy, Sweden, Germany, the Netherlands, Australia and the U.S. In our recommendations we focus on the first four steps of the value chain set out in Figure 1. By analysing the current state-of-play in each country, we propose an aspirational governance framework that could guide the management of data access and use, and facilitate constructive interactions among the relevant stakeholders.
Governance arrangements for health data have at their core the need to balance public and privacy interests, of advancing our understanding of medical treatments through evaluation and research, on the one hand, and protecting individuals’ privacy, on the other. Different countries have different ways of addressing this aim. In most cases the legal framework in place to protect patient data is not completely prescriptive, making a clear governance framework all the more important.
We find that appropriate and facilitative information governance, along with public trust, is key to realising the benefits of scientific research. Along with sophisticated and transparent approaches to data linkage, patient consent has a key role to play, alongside mechanisms for anonymisation and authorisation. For example, where data collection is on a routine basis across a large patient cohort, an opt-out system of patient consent may be appropriate. Data can be de-identified by removing any personally identifiable information and replacing the unique patient identifier with a pseudonym.
By outlining the individual aspects and actions of an ideal framework for data governance, the authors assess the extent to which each of the countries analysed currently meets these criteria. The resulting set of recommendations work towards setting out international standards for a more facilitative environment for the transformation of RWD into RWE.
Access the full report here.
For more information contact Amanda Cole at OHE.
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!