Sign up to our newsletter Subscribe
Analysing Global Immunisation Expenditure

Sign up to our newsletter Subscribe


It is estimated that one in three people born in 2015 in the UK may develop dementia, and clinical development success rates for dementia drugs are consistently lower than those for other therapy areas. A new analysis conducted by OHE’s…
It is estimated that one in three people born in 2015 in the UK may develop dementia, and clinical development success rates for dementia drugs are consistently lower than those for other therapy areas.
A new analysis conducted by OHE’s Dr Fraser Lewis estimates that one in three people born in 2015 in the UK will develop dementia. A second OHE analysis, conducted by Grace Marsden and Dr Jorge Mestre-Ferrandiz, looks at dementia treatments currently in clinical development, and reveals that clinical development success rates for dementia drugs are consistently lower than those for other therapy areas (a finding consistent with other similar analyses done to date).
The estimates of future cases of dementia are a result of work commissioned by Alzheimer’s Research UK. The estimates were based on three components:

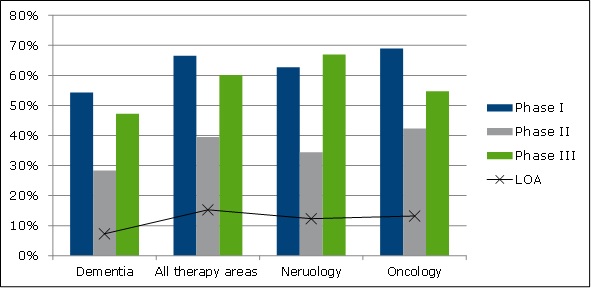
Phase success rate calculations revealed that dementia indications have lower success rates than other therapy areas (see Figure 2).
Figure 2: Phase success probabilities
An error has occurred, please try again later.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!

